Understanding the Electrochemical Oxidation Pathways of Acetaminophen Removal
Summary
This article not only discuss about some information about the structures, and properties of acetaminophen, and how it affects the aqua system, but also covers current treatment method applied to remove acetaminophen, and the fundamentals of electrochemical destruction of acetaminophen .
Advanced electrochemical oxidation is a proven and cost-effective treatment method for the destruction of acetaminophen. In its most fundamental application and testing process, the advanced electrochemical oxidation process here, anodic oxidation to be precise, involves applying an electrical current across a pair of non-active boron-doped diamond electrode plates immersed in the process stream.
The actual degradation mechanisms and processes, intermediates involved in advanced electrochemical oxidation treatment are the focus of this article.
Introduction to Acetaminophen
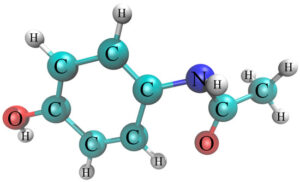
*chemical structure of Acetaminophen(ACT)
In recent decades, there has been a significant increase in the consumption of pharmaceuticals and personal care products (PPCPs) .
Acetaminophen (ACT, N-(4-hydroxyphenyl) is a commonly used drug among pharmaceuticals and personal care products (PPCPs) with significant rise in the consumption dose worldwide, due to inefficient absorption and incomplete metabolism, most of the acetaminophens are discharged into the environment in their raw form or intermediates. These compounds (Acetaminophen and its intermediates) are frequently detected in aqueous environments, leading to their emergence as contaminants due to their adverse effects.
Acetaminophen, also known as paracetamol, is a versatile medication that provides relief from a variety of common ailments, is a widely used over-the-counter(OTC) pain reliever and fever reducer, it’s one of the essential medications readily available to individuals seeking relief from pain and discomfort, acetaminophen is a significant segment within the pharmaceutical industry, dedicated to the production and distribution of acetaminophen.
One of the primary driving factors for acetaminophen production and consumption is the widespread use of acetaminophen as a safe and effective pain management option. It is a trusted choice for relieving mild to moderate pain and reducing fever in individuals of all ages.
Acetaminophen’s reputation for safety and minimal side effects has made it a preferred option for many healthcare professionals and patients. Acetaminophen somehow become an accessible and reliable pain relief solution which ensure that people can manage their discomfort and fever effectively.
Additionally, the increasing prevalence of conditions that cause pain and fever is contributing to the growth of the Acetaminophen market. Factors such as aging populations, the rise of chronic pain conditions, and the occurrence of febrile illnesses drive the continuous demand for pain relief and fever-reducing medications , and given its versatileness and aptness for patients within different ages and medical conditions making it a vital tool in healthcare, driving market expansion.
Acetaminophen (ACT) is considered a highly persistent organic pollutant (POPs) with uncertain and long-term effects on human and animal health. Despite a low ACT concentration, long-term exposure will cause a constant accumulation of toxicological effects, thereby inducing irreversible damage to human health and the ecosystem. For instance, physiological and morphological alterations in zebrafish have been widely reported in recent years. Endocrine disruption and chronic diseases (including gastrointestinal, cardiovascular, and kidney diseases) may be also caused in humans by denaturing the proteins, damaging the genetic code, and oxidizing the lipids due to long-term exposure even at trace acetaminophen concentration.
Acetaminophen Removal Method Advancements & New Approach
The hazardous and toxic property of contaminants such as acetaminophen and its intermediates poses a serious threat to organisms and the ecosystem. Therefore it’s necessary to explore and develop effective wastewater treatment technology to remove organic pollutants like acetaminophen and its intermediates in aqueous environments. While traditional wastewater treatment technologies play a crucial role in controlling water pollution, they are flawed in several limitations, including high energy consumption, low treatment efficiency, and the potential for secondary pollution. Consequently, it is crucial to develop wastewater treatment technologies that exhibit low energy consumption, high removal efficiency, and wide applicability to effectively address these issues.
As for ACT and its metabolites, extensive research has been carried out to address their removal from wastewater. However, it is reported that conventional wastewater treatment technologies, including activated sludge, biodegradation, ozonation, and filtration, in wastewater treatment plants (WWTPs) are still far from satisfactory concerning the removal of ACT.
For instance, multiple bacterial strains were isolated from pharmaceutical effluents and used for the biodegradation of ACT, and the highest degradation efficiency was 92.35% by Pseudomonas strain PrS10 after 7 days treatment. The most efficient technologies developed for this purpose are the advanced oxidation processes (AOPs), which are environmentally friendly methods relying on the oxidative degradation of organic pollutants by generating reactive oxygen species (ROS), especially hydroxyl radicals.
Nevertheless, when the pollutants are diluted in large quantities, the application of typical AOPs (electrocatalysis) will become technically and economically difficult. Among various AOPs, advanced electrochemical oxidation (anodic oxidation) technology has overcome the usual rejection of AOPs and has gained significant attention in removing ACT due to its excellent degradation efficiency, low cost, and the potential for the sustainable solar-induced degradation of refractory pollutants.
In the following content, the electrocatalysis advanced oxidation technology for effective removal of ACT from aqueous solutions by different kinds of electrocatalysis is comprehensively introduced. This study is organized into four parts to provide a comprehensive analysis. The first part presents essential information on the property and consumption of ACT. The second part examines the occurrence of ACT in various aqueous environments, including surface water, groundwater, and wastewater, followed by an overview of the potential impact of ACT on the ecosystem. In the third part, the focus is on the removal of ACT using electrocatalytic AOPs. Specifically, it investigates the effectiveness of different electrocatalysis materials in eliminating ACT from aqueous environments, including metal and non-metal doping, boron-doping electrocatalysis.
Finally, the fourth part discusses the primary challenge and future trends of applying electrocatalytic AOPs in practical ACT treatment. This section provides a comprehensive overview of the electrocatalytic degradation of ACT in aqueous environments.
As concerns surrounding Acetaminophen(ACT) intensify, the waste management and landfill industries face mounting challenges. Regulatory standards are tightening, necessitating innovative solutions for acetaminophen destruction. Electrochemical oxidation emerges as a promising method. we delve into the details of electrochemical oxidation, exploring its mechanisms and practical applications in acetaminophen remediation.
Electrode and Catalyst Choice Matters
As the title of this specific part suggests, electrode material is crucial in the design of any electrochemical treatment process, with a vast variety of electrode materials available to choose from. In most cases, the electrode material consist of a conductive metal sutrate coated with a more highly reactive catalyst material. From lowest to highest reactivity, materials such as tin or lead, precious or rare earth metals (platinum, iridium, etc.), mixed metal oxides, titanium sub-oxides, or boron-doped diamond (BDD) are all options for the treatment processes. Each catalyst material determines both the type and rate of chemical reactions available in the treatment process.
Although the inclination might be to choose the most reactive electrode materials, other factors are critical to consider. One of the important factors to consider is the service life and replacement interval of the electrodes, as all catalyst materials have a finite service life and will require regular replacement. While replacement intervals vary based on the application and manufacturer, a service life of two–five years can be expected. Additionally, the electrical efficiency of each material type impacts the operating cost of the system. Balancing service life, replacement cost, and operating cost are crucial for each project. Systems employing a mix of multiple electrode materials usually yield the most effective solution, in practical trial scale testing, we employed boron doped diamond electrode as anode and titanium electrode as the cathod within the electrochemical oxidation process.
Reactions for Acetaminophen Destruction
Electrochemical oxidation is an iteration of advanced oxidation processes (AOPs) increasingly applied in water treatment. The mechanisms of electrochemical oxidation of acetaminophen involve complex and competitive reaction pathways influenced by factors such as electrode material, electrolyte composition, pH, temperature, applied electrical potential, and reaction kinetics. The key distinction with electrochemical oxidation is its ability to produce high oxidation energy and favorable conditions to promote acetaminophen destruction.
In practice, acetaminophen destruction involves successively breaking longer-chain acetaminophen compounds into short-chain molecules.

The complexity of the oxidation process is illustrated in the oxidation destruction pathways for acetaminophen, as shown in Figure 2. At the anode, acetaminophen molecules undergo oxidative degradation primarily through direct electron transfer reactions. Indirect oxidation occurs using oxidant compounds such as hydroxyl radicals (*OH) generated at the electrode surface simultaneously. The powerful oxidative conditions created in the electrochemical process allow these reactions to proceed until complete mineralization to elemental precursors are all that remain, such as CO2 gas, H2 gas, fluoride ions, and other mineral salts. Simple pH control is used to prevent the production of hydrogen fluoride (HF) in the destruction process.
Practical Applications
Research into electrochemical oxidation of acetaminophen has focused on optimizing reaction conditions, exploring new electrode materials, exemplifying working mechanisms, and assessing the environmental fate of degradation products. Recent studies have demonstrated the effectiveness of electrochemical oxidation for the treatment of acetaminophen-contaminated streams, achieving high removal efficiencies and generating non-toxic byproducts. Challenges remain, including developing cost-effective electrode materials with enhanced catalytic activity, minimizing energy consumption, and mitigating potential risks associated with the formation of transformation by-products.
Evidence that electrochemical oxidation occurs through successive oxidation is easy to see by examining the relative concentration of acetaminophen compounds at regular intervals throughout the treatment process. Treatment results for an industrial wastewater sample containing over 30 mg/L of various acetaminophen compounds and over 46,000 mg/L of COD. Acetaminophen molecules are successively oxidized to produce intermediate compounds, continuing until compounds are completely mineralized. In the example shown, over 98.7% destruction of all acetaminophen compounds was achieved, with removal of most compounds exceeding 99.9%.
The Importance of Testing
The breadth and scope of acetaminophen treatment challenges in environment make every application unique. While the electro oxidation processes described apply to every application, the specific compounds to be treated and water chemistry of every project are unique. To address these issues, lab and pilot-scale testing for each application are critical for project success. Laboratory testing helps the process design team identify the correct mix of electrode and catalyst materials for the most favorable oxidation conditions. Subsequent pilot testing optimizes the overall operating conditions for the treatment process. This 1-2 testing approach ensures that complete acetaminophen destruction can be achieved cost-effectively.
Offering advantages in efficiency, selectivity, and environmental compatibility, electrochemical oxidation represents one of the most promising approaches for acetaminophen destruction in contaminated water sources. The powerful oxidizing conditions created and sustained in the process ensure that the full spectrum of acetaminophen molecules can be destroyed without formation of harmful by-products. Customers taking a systematic approach to evaluating their application can identify the best materials and operating conditions to ensure cost-effective treatment and guaranteed compliance with rapidly evolving regulatory requirements for PFAS.
A Proven Solution
In an era marked by stringent regulatory frameworks and escalating acetaminophen contamination, the need for effective remediation methods is paramount. Electrochemical oxidation stands at the forefront, offering a blend of efficiency, flexibility, and reliable destruction results. As industries navigate evolving compliance standards, a systematic approach to evaluating electrochemical oxidation can pave the way for cost-effective and sustainable acetaminophen treatment solutions. Through ongoing research and practical application, electrochemical oxidation continues to redefine the landscape of acetaminophen remediation, ensuring a cleaner and safer environment for future generations.
About Evoaeo
Evoaeo is a professional engineer with more than 5 years of experience in water and wastewater treatment, including advanced oxidation and electrochemical oxidation processes. During this time, he has been involved in delivering treatment solutions and commercializing treatment technologies in China, and expanding all over the world.
Connect with Evoaeo on LinkedIn, via email at enquiry@evoaeo.com.
Look out for our future articles on electrochemical Acetaminophen destruction in upcoming issues.
For more information about Evo Technology’s advanced electrochemical oxidation process, visit www.evoaeo.com.